The Mohr's salt is shown by Chemistry Questions

3. Compute Mass of Each Element. Multiply the number of atoms by the atomic weight of each element found in steps 1 and 2 to get the mass of each element in Fe (NH4)2 (SO4)2*6H2O: Molar Mass (g/mol) Fe (Iron) 1 × 55.845 = 55.845. N (Nitrogen) 2 × 14.0067 = 28.0134. H (Hydrogen)
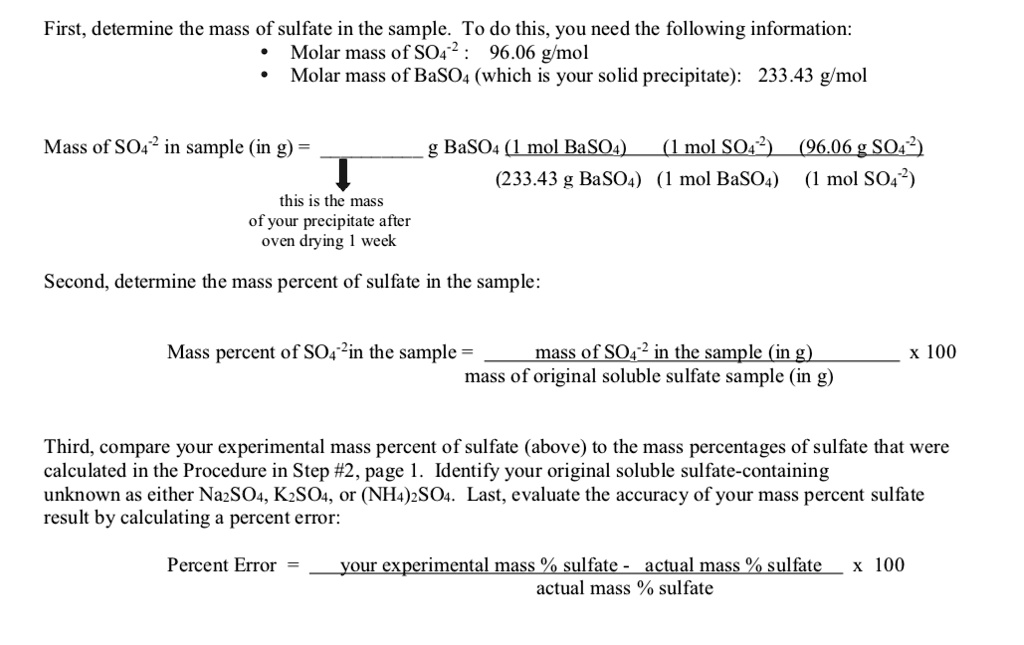
SOLVED First, determine the mass of sulfate in the sample. To do this, you need the following
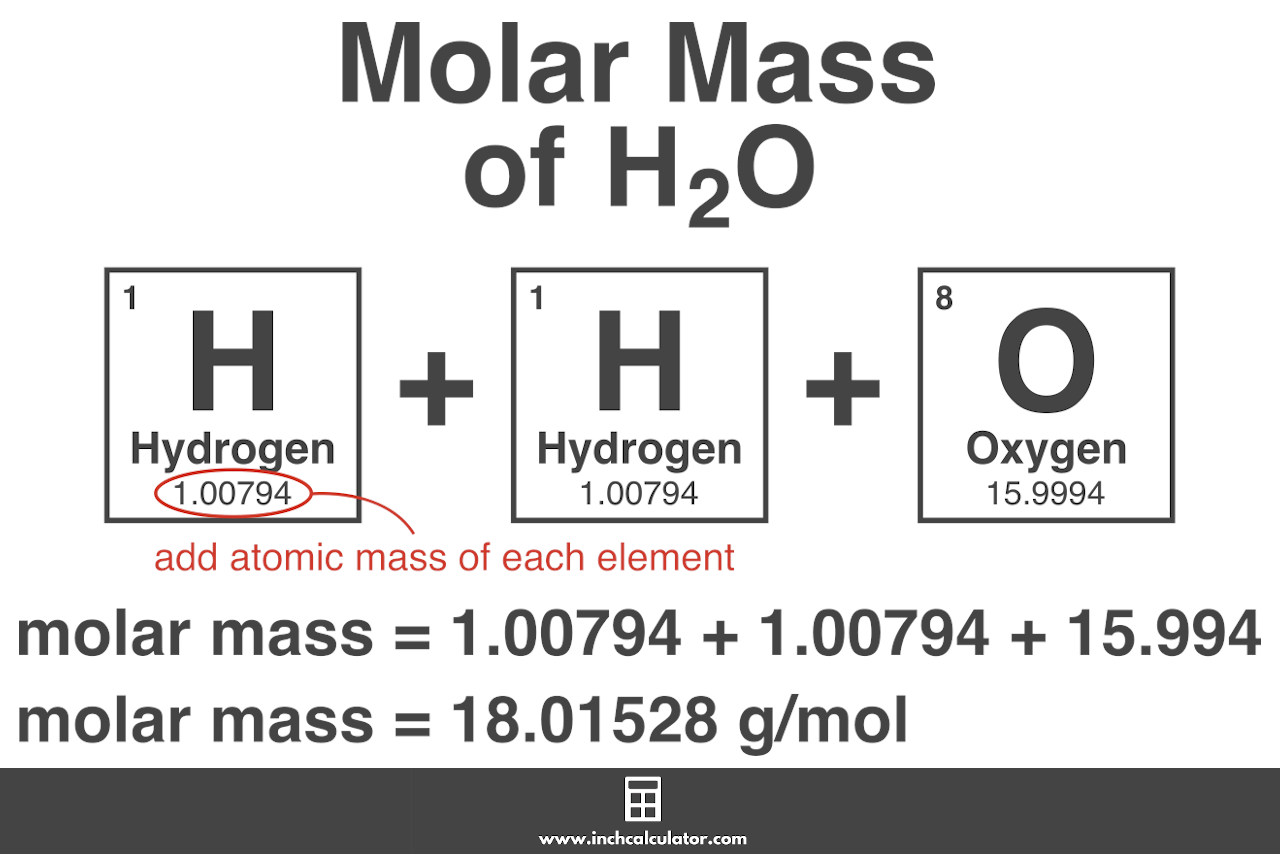
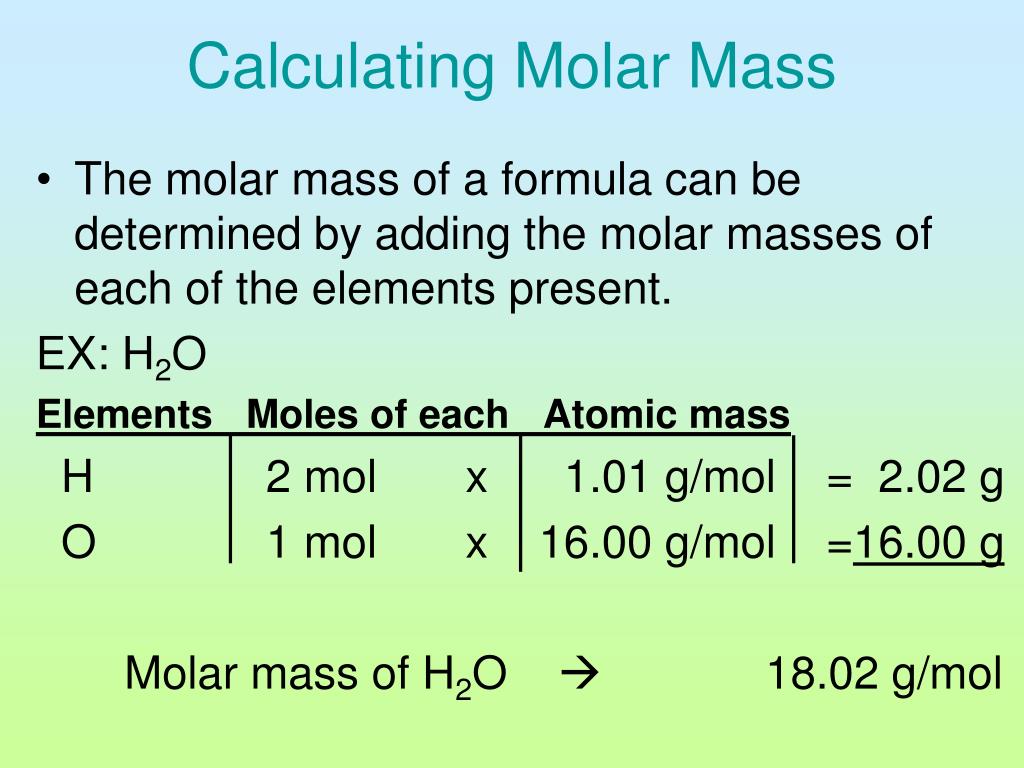
Add them together: add the results from step 3 to get the total molar mass of the compound. Example: calculating molar mass. Let's calculate the molar mass of carbon dioxide (CO 2): Carbon (C) has an atomic mass of about 12.01 amu. Oxygen (O) has an atomic mass of about 16.00 amu. CO 2 has one carbon atom and two oxygen atoms.
Muối Mohr (NH4)2Fe(SO4)2 Amonium Iron II sulfate hexahydrate
2FE(SO4)2-MUOI-MOHR-XILONG-TQ (1).jpg)
This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Question: Calculate the mass of Fe (NH4)2 (SO4)2 * 6H2O was present in your unknown sample. Neatly show your work. Calculate the mass of Fe (NH4)2 (SO4)2 * 6H2O was present in your unknown sample. Neatly show your work.
from the following molar conductivities at infinite dilution molar conductivity of AL2(SO4)3
Part A: Standardization of Permanganate Determination 1 Determination 2 0.30 g (50ml H2O) Data Mass of weighing paper, g (if using) Mass of weighing paper + (NH4)2Fe (SO4)2.6H20, g Mass of (NH4)2Fe (SO4)2-6H20,8 Initial buret reading, mL Final buret reading, mL Volume of permanganate, mL Results: 0.26 g (50ml H2O) Oml 7.3 ml 7.3 ml 6.7 ml Number of moles of (NH4)2Fe (SO4)2-6H20, mol Number of.
SOLVED How many hydrogen atoms are there in 7.50g of (NH4)2Fe(SO4)2 · 6H2O?
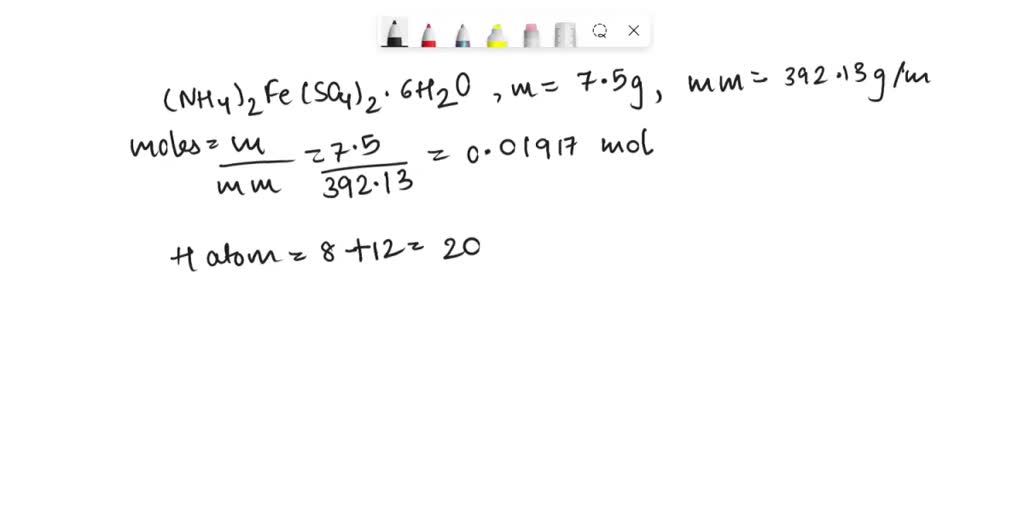
Ammonium iron (II) sulfate hexahydrate MSDS (material safety data sheet) or SDS, CoA and CoQ, dossiers, brochures and other available documents. SDS. CoA. Brochures. CAS #: 7783-85-9 EC Number: 233-151-8 Molar Mass: 392.14 g/mol Hill Formula: H₈FeN₂O₈S₂*6H₂O Grade: ISO. View Products on Sigmaaldrich.com. 103792.
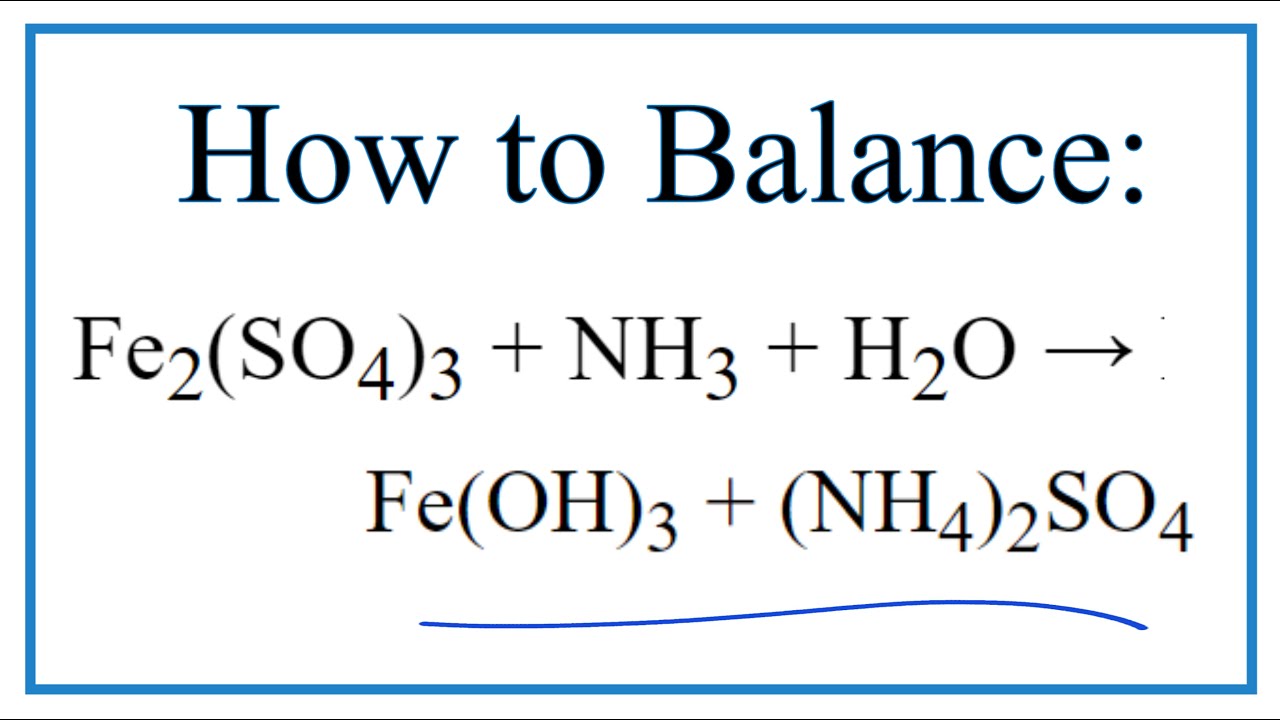
How to Balance Fe2(SO4)3 + NH3 + H2O = Fe(OH)3 + (NH4)2SO4 YouTube
Step 1. The mole fraction including t. What is the molar mass of Fe (NH_4)_2 (SO_4)_2 6H_2O (s) Using the molar mass of Fe (NH_4)_2 (SO_4)_2 middot 6H_2O (s) and the amount used in your experiment. 1 calculate the number of moles of Fe (NH_4)_2 (SO_4)_2-6H_2O (s) you used in this experiment. According to the balanced chemical reaction, for.
How to Write the Net Ionic Equation for (NH4)2SO4 + NaOH = Na2SO4 + NH3 + H2O YouTube

Iron(II) Ammonium Sulfate (NH4)2Fe(SO4)2.6H2O Molecular Weight, molar mass converter.. (NH4)2Fe(SO4)2.6H2O is a blue green crystal at room temperature. It is soluble in water. Its melting point is 100 ̊C (212 ̊F), density 1.86 g/cm3. Mohr's salt may cause burns to eye and skin. It may cause digestive tract irratation if swallowed.
Mohrite((NH4)2Fe(SO4)2.6H2O) (9CI) 24389933 wiki

Molar mass of (NH4)2Fe (SO4)2 is 284.0471 g/mol. Get control of 2022! Track your food intake, exercise, sleep and meditation for free. Convert between (NH4)2Fe (SO4)2 weight and moles. Compound. Moles. Weight, g.
molar mass of feso4(nh4)2so4*6h2o Chemistry Some Basic Concepts of Chemistry 13704168

Molar Mass, Molecular Weight and Elemental Composition Calculator. Enter a chemical formula to calculate its molar mass and elemental composition: Molar mass of (NH4)2Fe (SO4)2 (H2O)6 is 392.1388 g/mol. Get control of 2022! Track your food intake, exercise, sleep and meditation for free. Convert between (NH4)2Fe (SO4)2 (H2O)6 weight and moles.
Natri Sulfate (AR) (NH4)2Fe(SO4)2.6H2O Vật Tư Bách Khoa

Ammonium iron(II) sulfate, or Mohr's salt, is the inorganic compound with the formula (NH 4) 2 Fe(SO 4) 2 (H 2 O) 6.Containing two different cations, Fe 2+ and NH + 4, it is classified as a double salt of ferrous sulfate and ammonium sulfate.It is a common laboratory reagent because it is readily crystallized, and crystals resist oxidation by air.
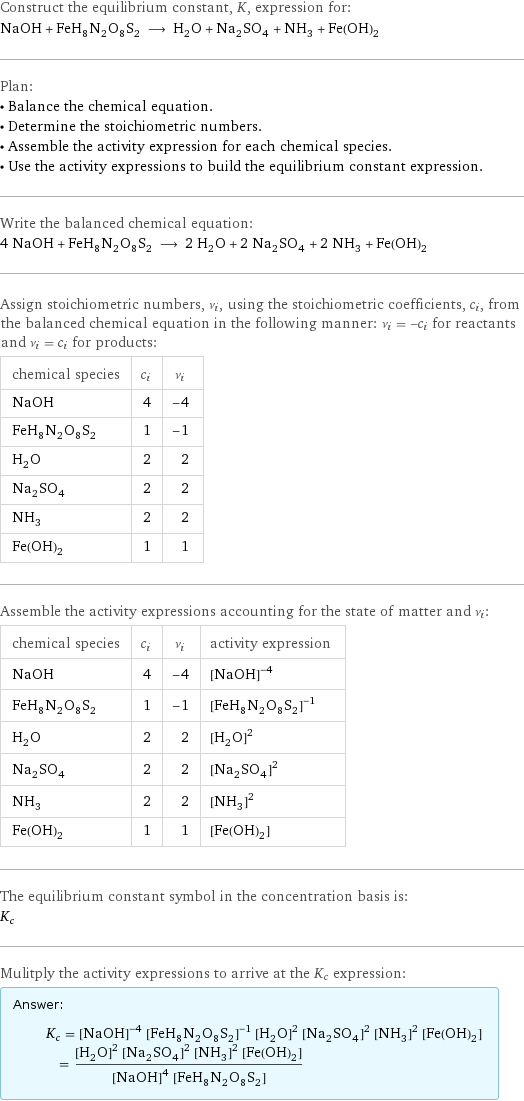
NaOH + (NH4)2Fe(SO4)2 = H2O + Na2SO4 + NH3 + Fe(OH)2
Example: calculating molar mass. Let's calculate the molar mass of carbon dioxide (CO 2): Carbon (C) has an atomic mass of about 12.01 amu. Oxygen (O) has an atomic mass of about 16.00 amu. CO 2 has one carbon atom and two oxygen atoms. The molar mass of carbon dioxide is 12.01 + (2 × 16.00) = 44.01 g/mol. Lesson on computing molar mass
Solved (NH4)2Fe(SO4).6H2O + H2C2O4.2H2O → FeCO + (NH4)2SO4 +

Molar Mass of Ammonium Ferrous Sulfate: (NH4)2Fe(SO4)2 / Molecular Weight Calculator.. Molecular Weight Calculator Molar Mass of: Ammonium Ferrous Sulfate (NH4)2Fe(SO4)2 CAS: 10045-89-3. Formula Calculate Formatted Formula (NH 4)2Fe(SO 4)2 Empirical Formula. This application calculates MW for any chemical formula such as H2O,.
(nh4)2so4 Soluble Or Insoluble Draw Easy

See more Iron products. Iron (atomic symbol: Fe, atomic number: 26) is a Block D, Group 8, Period 4 element with an atomic weight of 55.845. The number of electrons in each of Iron's shells is 2, 8, 14, 2 and its electron configuration is [Ar] 3d 6 4s 2. The iron atom has a radius of 126 pm and a Van der Waals radius of 194 pm. Iron was discovered by humans before 5000 BC.
oxidation state of feso4 (nh4)2so4 6h2o Chemistry Redox Reactions 13222231

Formula:(NH4)2.(Fe.(H2O)6).(SO4)2 Enter a chemical formula to calculate molar mass,The molar mass calculator can be used in Chemical industry and medicine industry.. Molar Mass: 392.118 g/mol 1g=2.55025273004555E-03 mol Percent composition (by mass): Element Count Atom Mass %(by mass) N 2 14.007 7.14%.
Molar Mass Calculator Inch Calculator
Learn about ammonium iron (II) sulfate hexahydrate, a versatile reagent with applications in biochemistry, analytical chemistry and more.
Al molar mass htpastor
ferrous ammonium sulfate hexahydrate. ChEBI ID. CHEBI:76181. Definition. A hydrate that is the hexahydrate form of ferrous ammonium sulfate. Acts as an iron ion donor for building Fe-S clusters in vitro. Stars. This entity has been manually annotated by the ChEBI Team. Submitter.